AOAC approval for rapid test for Salmonella enteritidis
Neogen Corporation has announced that its rapid test for Salmonella enteritidis (SE) has received approval from AOAC International.
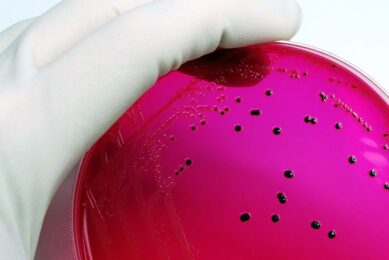
Neogen’s newly approved test kit, Reveal® for Salmonella enteritidis, provides the egg industry with a quick, accurate and easy method of detecting this pathogen, as the US Food and Drug Administration implements new SE-regulations. The AOAC’s process validated the accuracy of the Reveal for SE system when testing either environmental or egg samples.
“The AOAC’s approval further validates our test as an invaluable tool to egg producers, as they comply with the FDA’s new regulations, and seek to further reduce the likelihood of SE-contaminated eggs reaching consumers,” said Ed Bradley, Neogen’s vice president of Food Safety. “Until our introduction of an effective rapid test for SE, the industry had to wait up to 7 days for an outside laboratory’s test results. Reveal for SE enables the industry to get results within 48 hours — and provides the rapid, accurate answer they need to manage their flocks and egg production.”
Reveal for SE is the only rapid test available for SE that follows the FDA’s sample enrichment protocol and has received AOAC approval. Neogen, in cooperation with USDA scientists, began the development of this test in 2000.
Related website
Neogen
Join 31,000+ subscribers
Subscribe to our newsletter to stay updated about all the need-to-know content in the poultry sector, three times a week. Beheer
Beheer







 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht